AcT QuICR Trial
One of the first projects on our DCTMS platform, the AcT trial is a Pragmatic Phase III randomized open-label registry-based trial with blinded end-point assessment. The trial seeks to test whether intravenous tenecteplase (an intravenous thrombolytic drug tested and found safe in multiple phase 2 and one phase 3 trial is patients with acute ischemic stroke) can replace intravenous alteplase (the current standard care) in patients who are otherwise eligible to receive the latter in routine care.
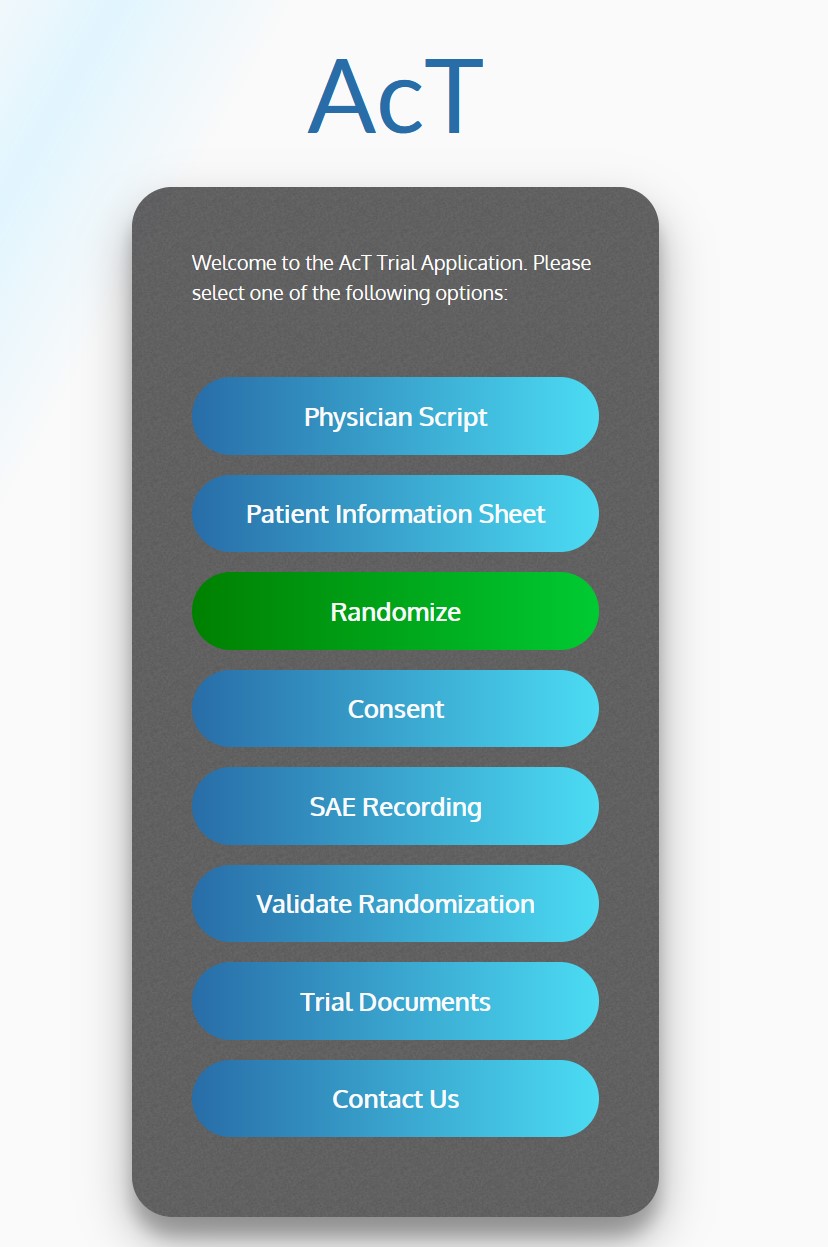
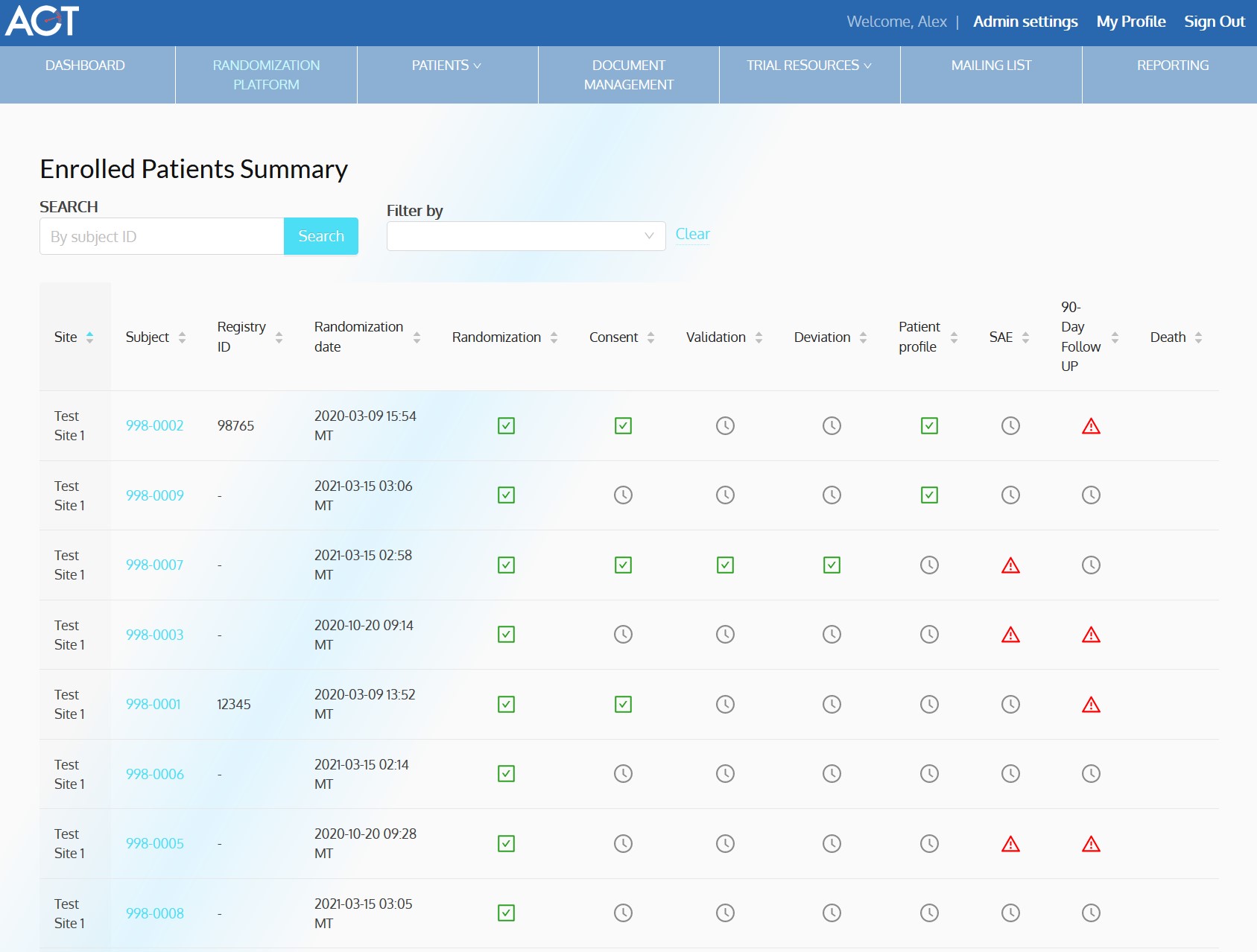
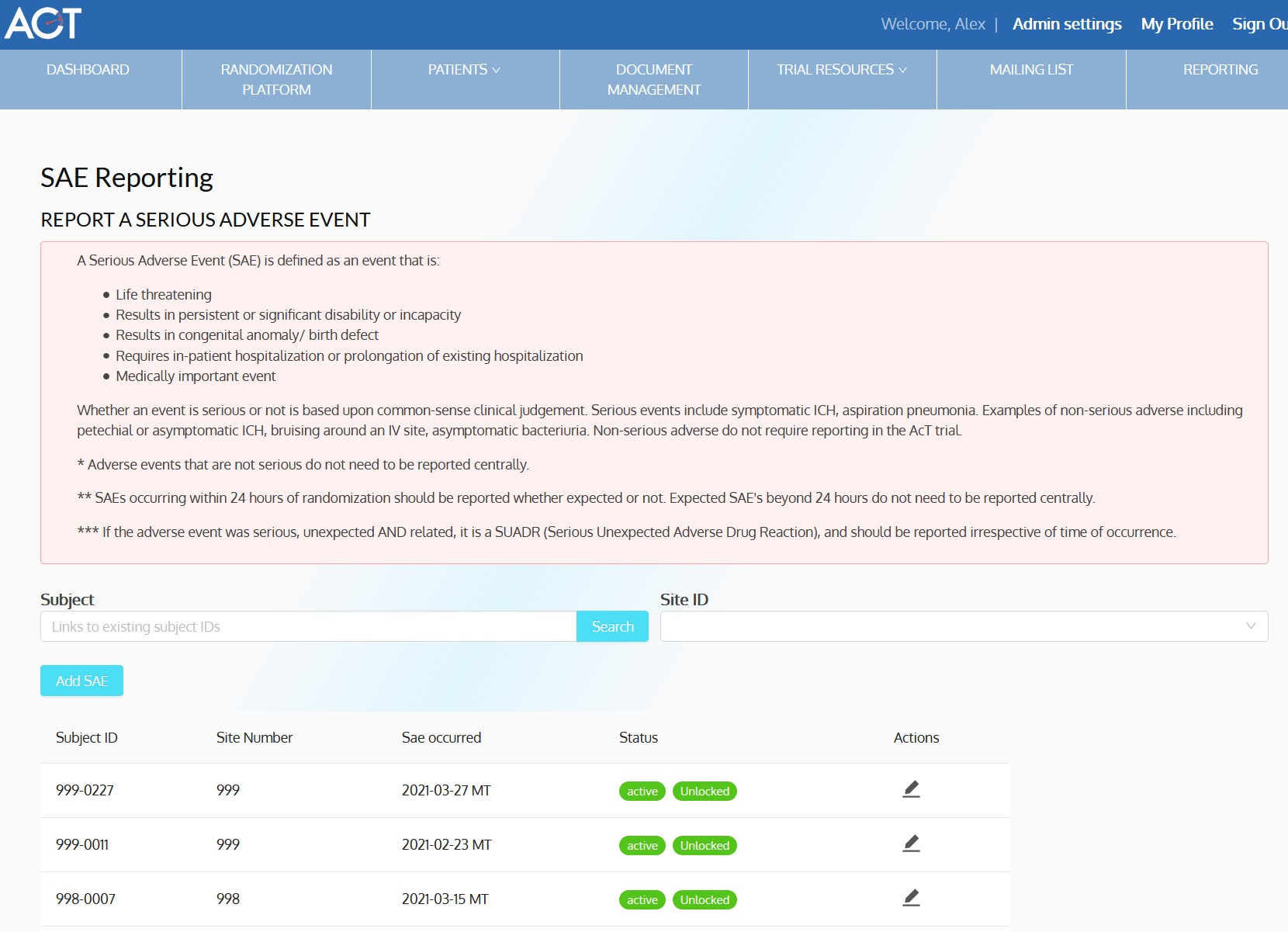
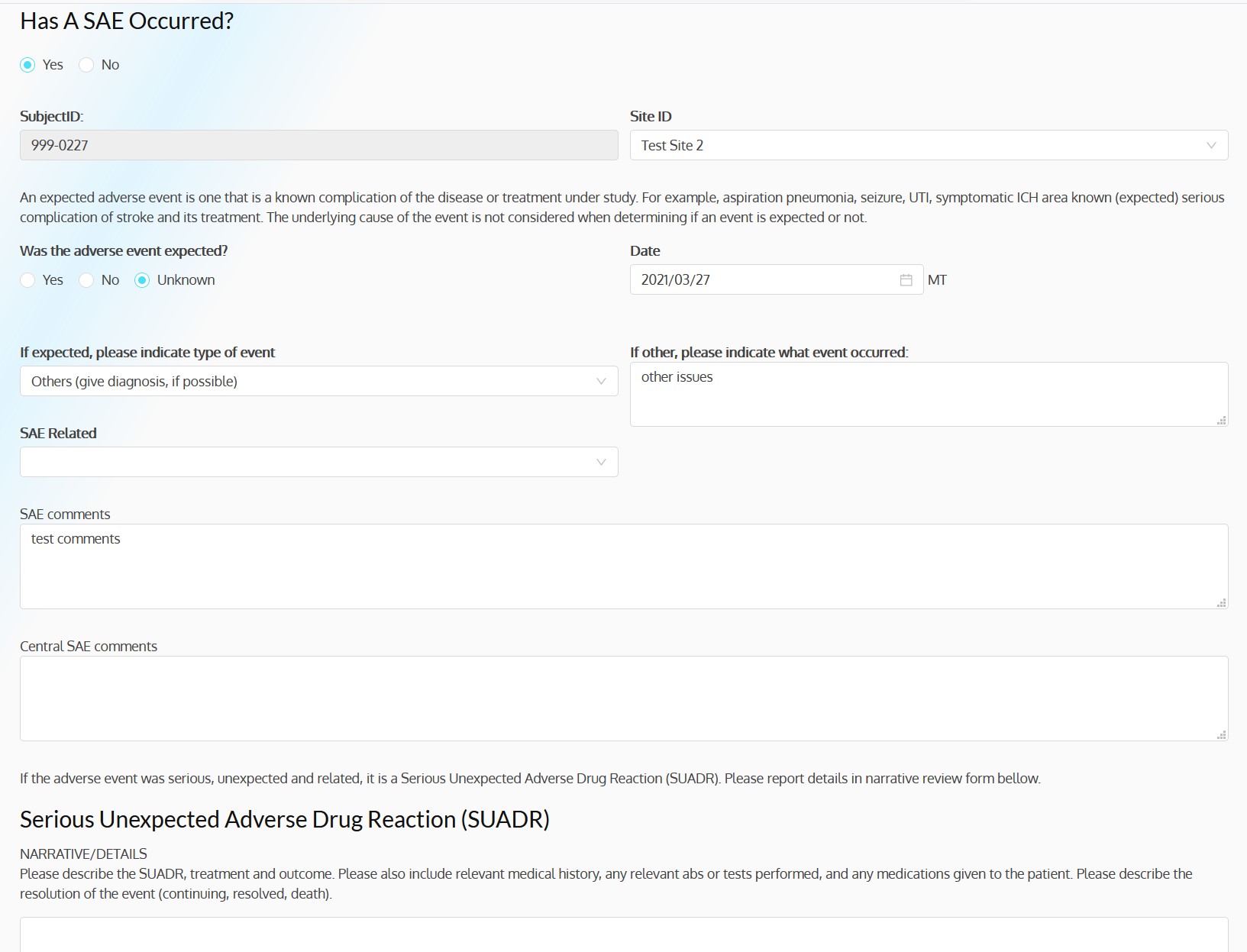
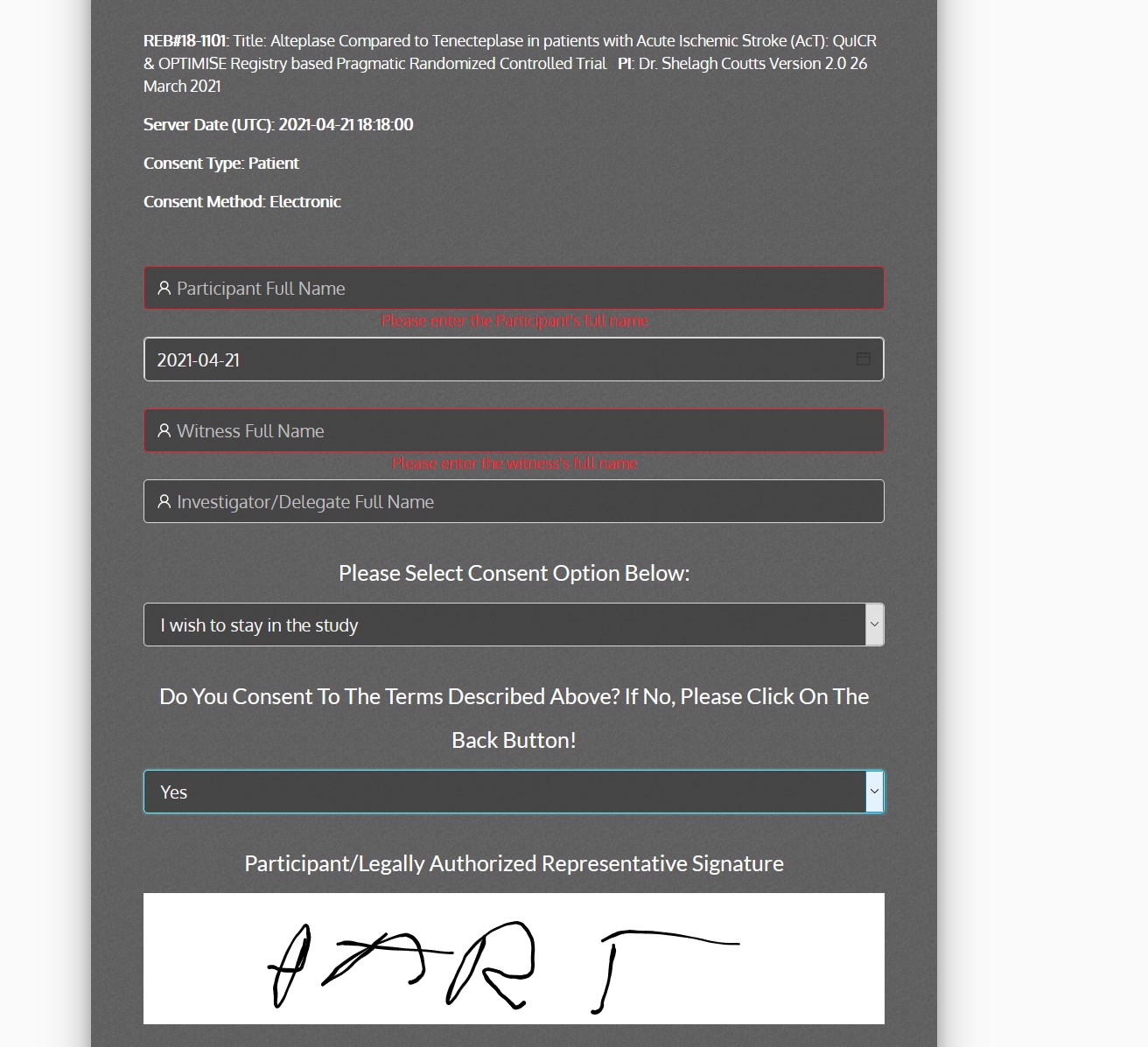
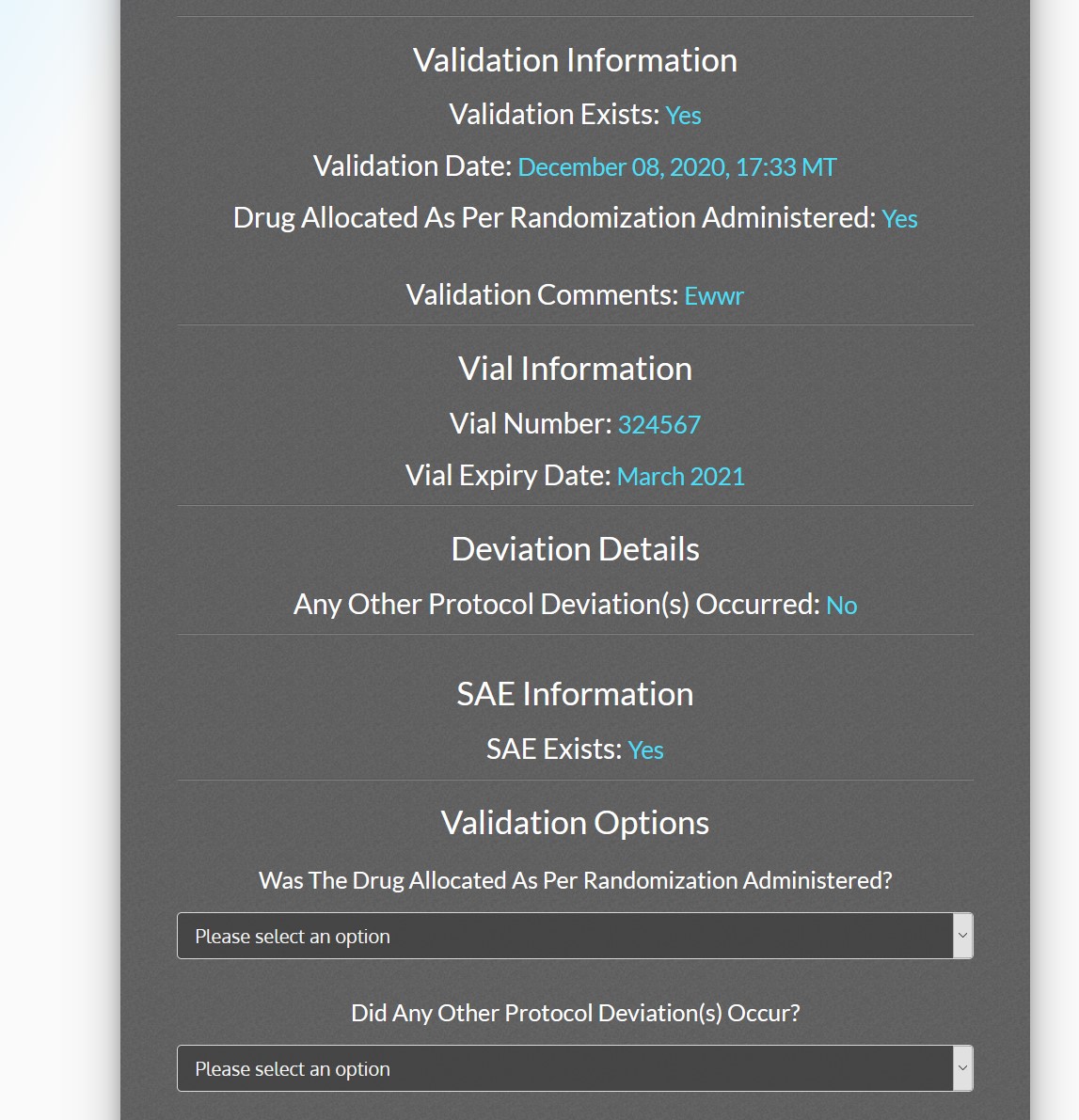
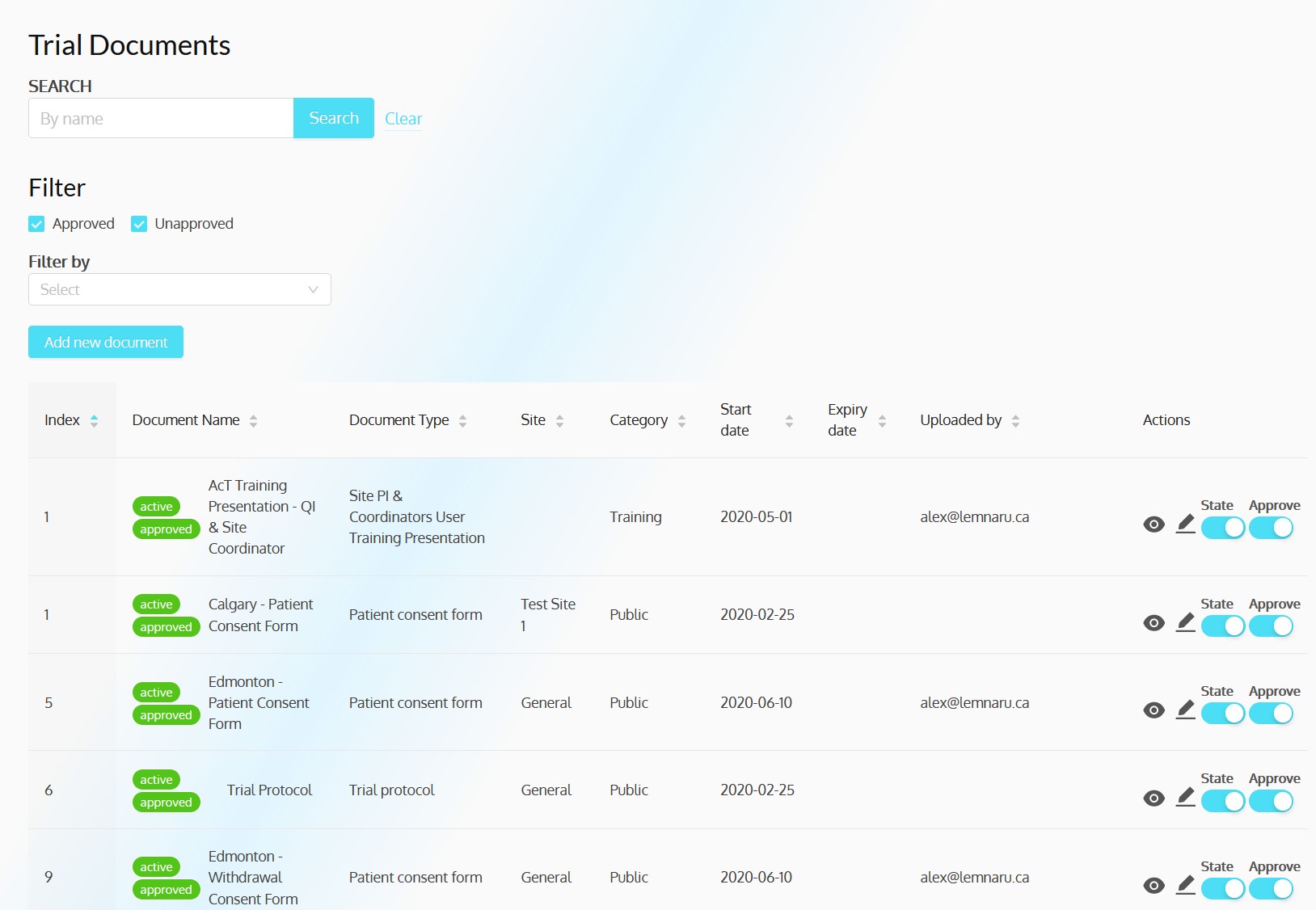
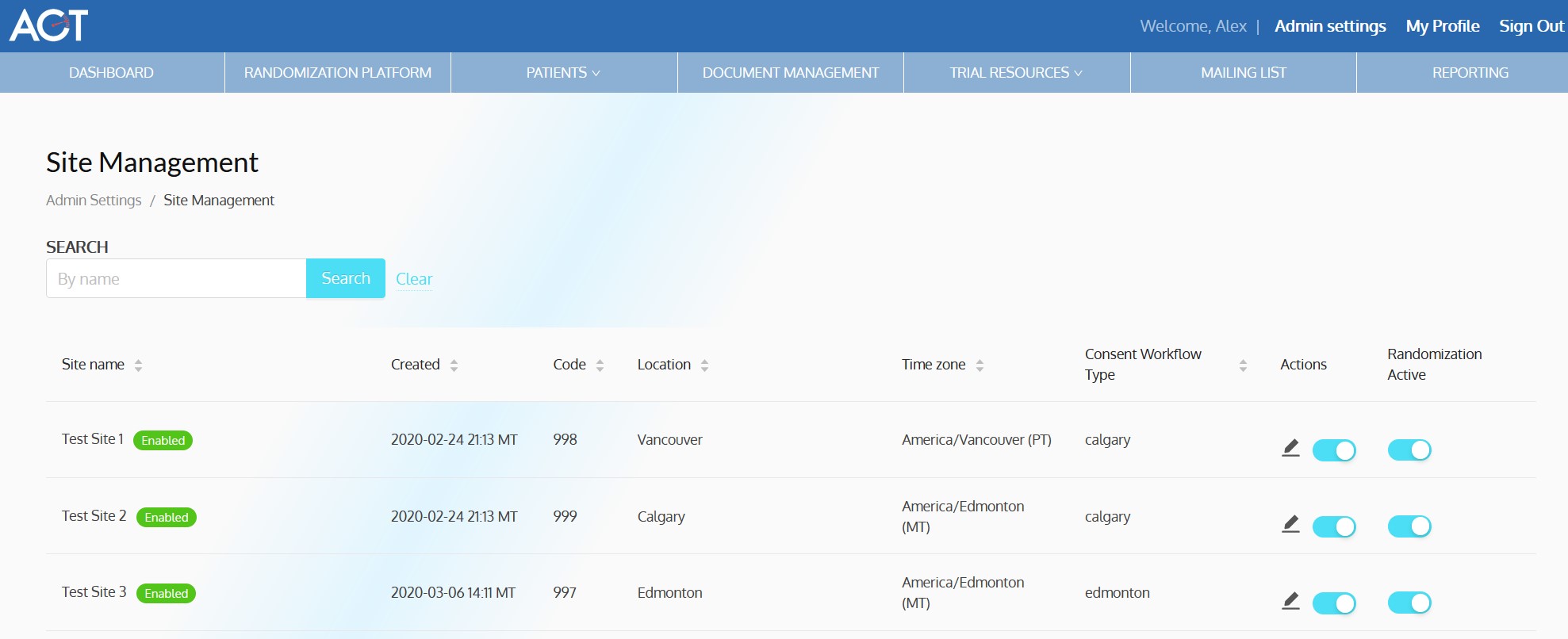
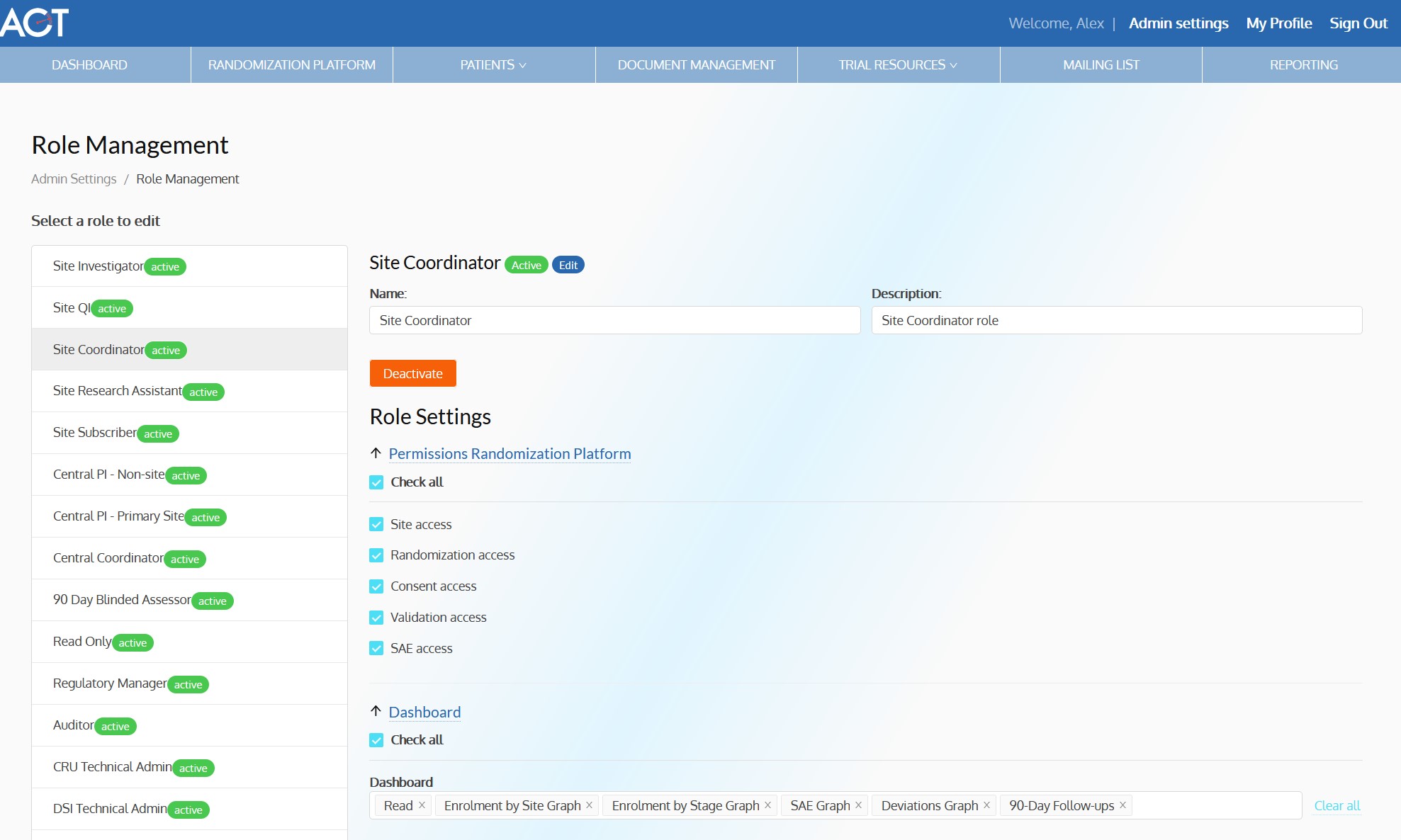
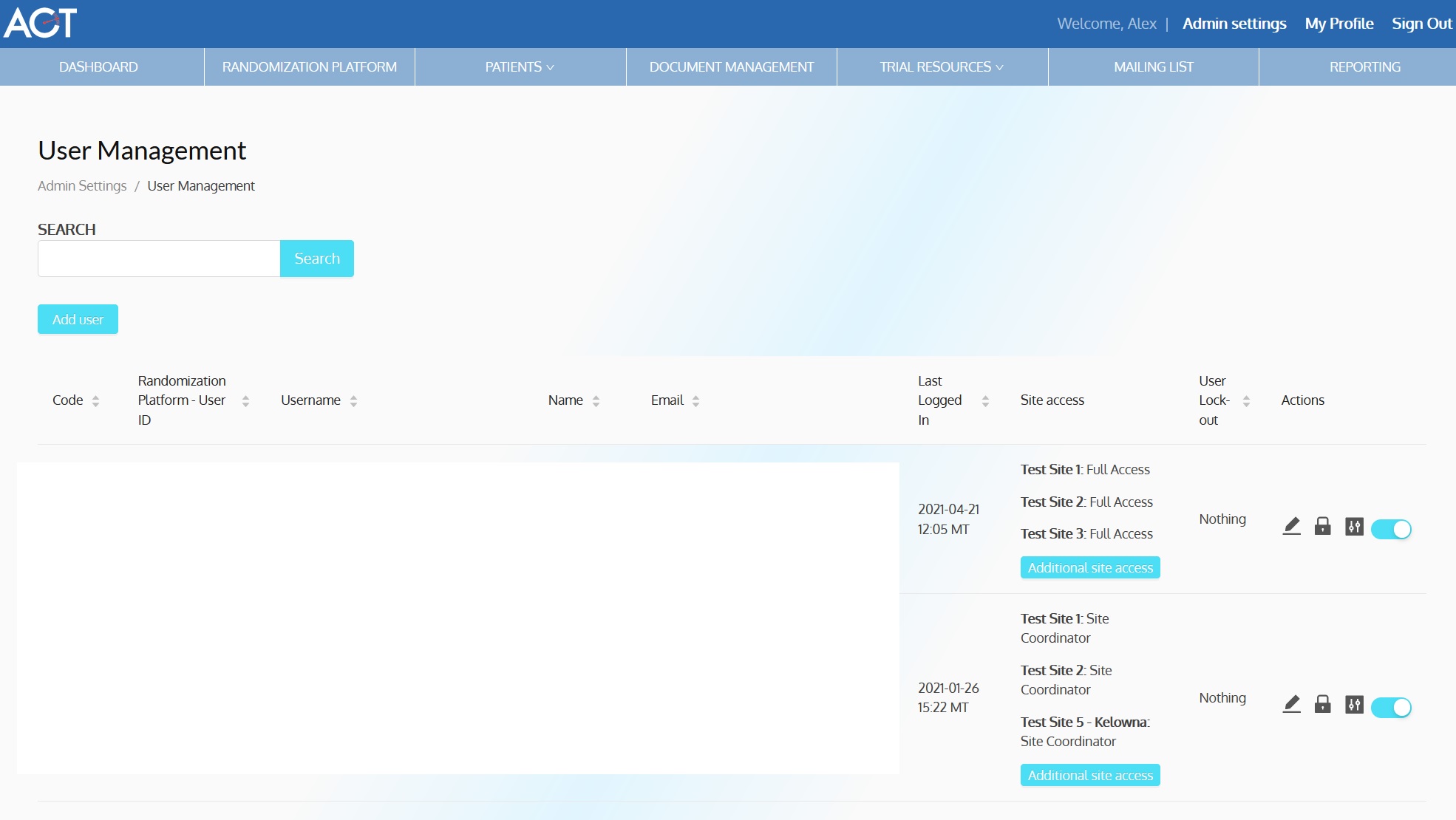
ACT-QUICR is a custom designed Electronic Data Capture (EDC) and Randomization Solution with the purpose of facilitating the data collection for the ACT-QUICR trial, while providing the following functions:
- Provides participating trial physicians with the physician script
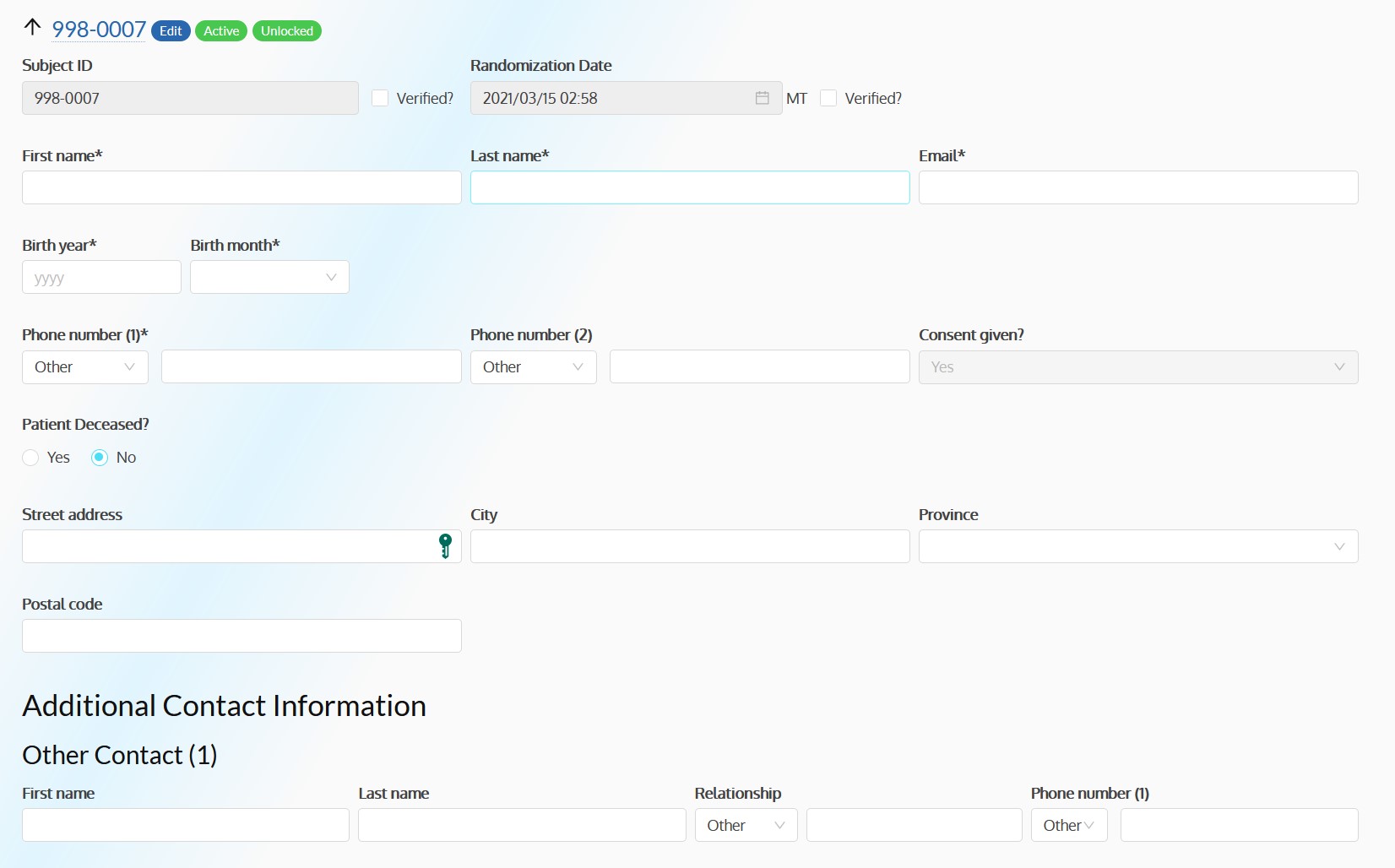
- Provides patients with relevant patient information
- Allow randomization of subjects based on the presented protocol
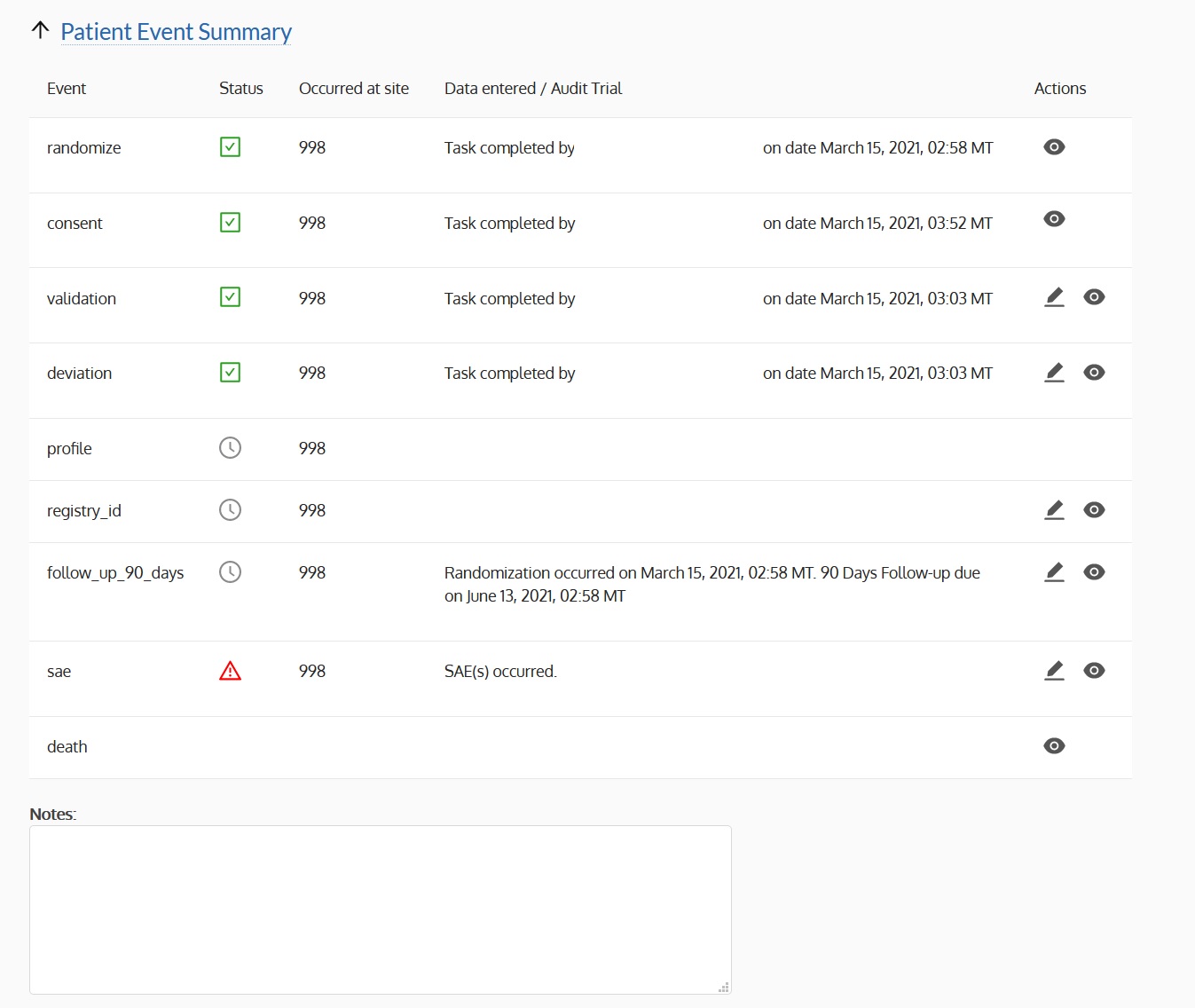
- Provide functionality for validating the randomization of subjects
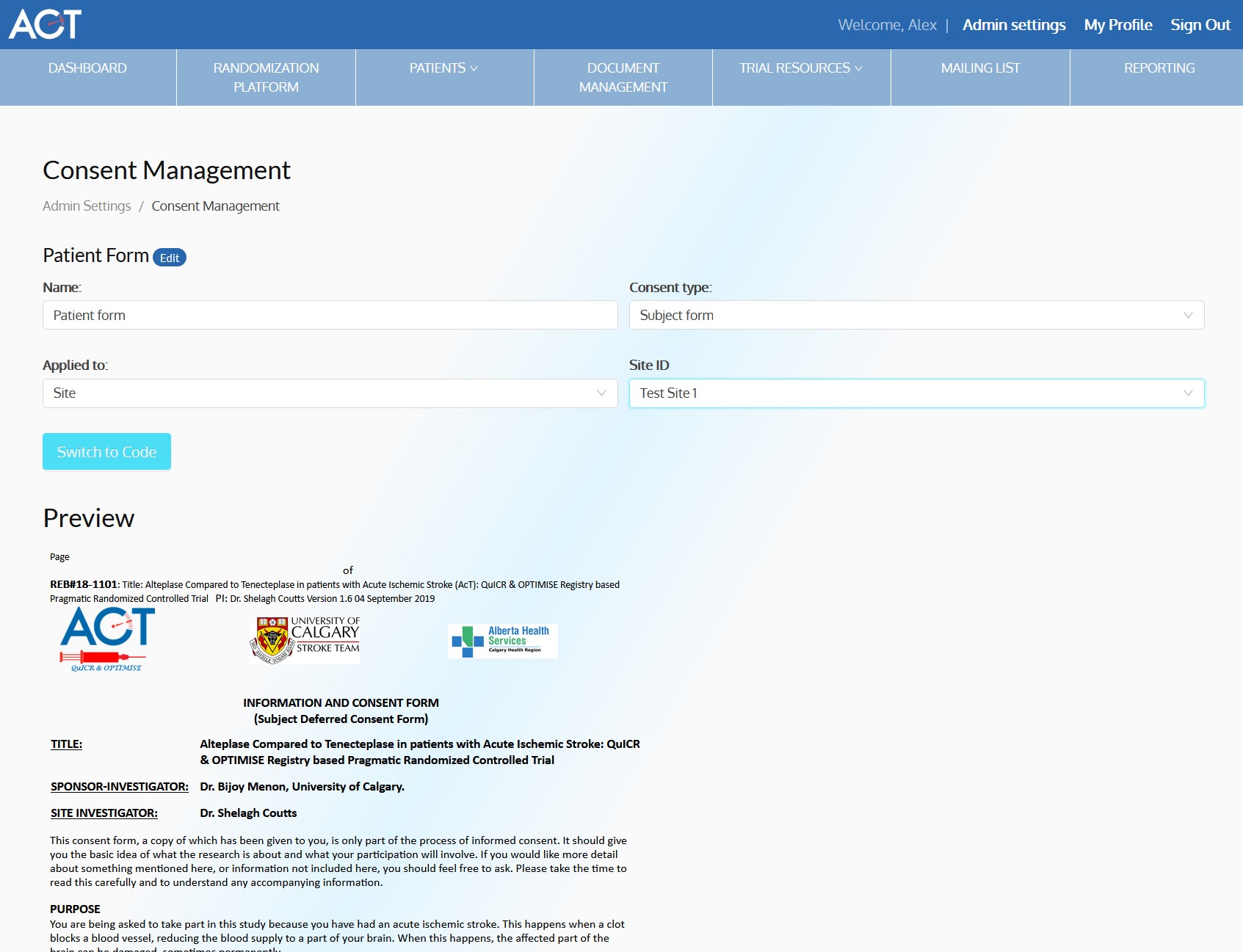
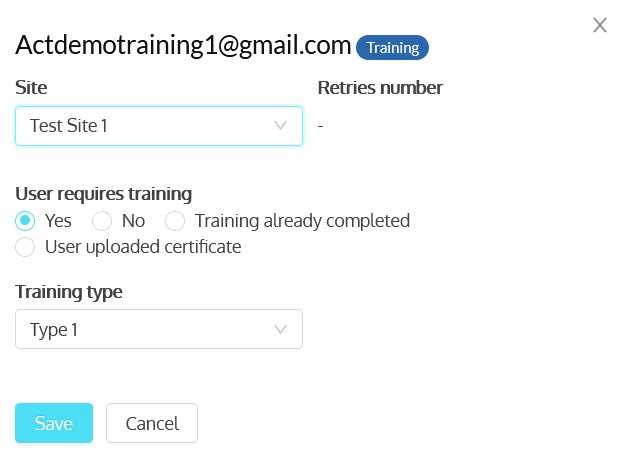
- Enable paper and electronic subject consent acquisition
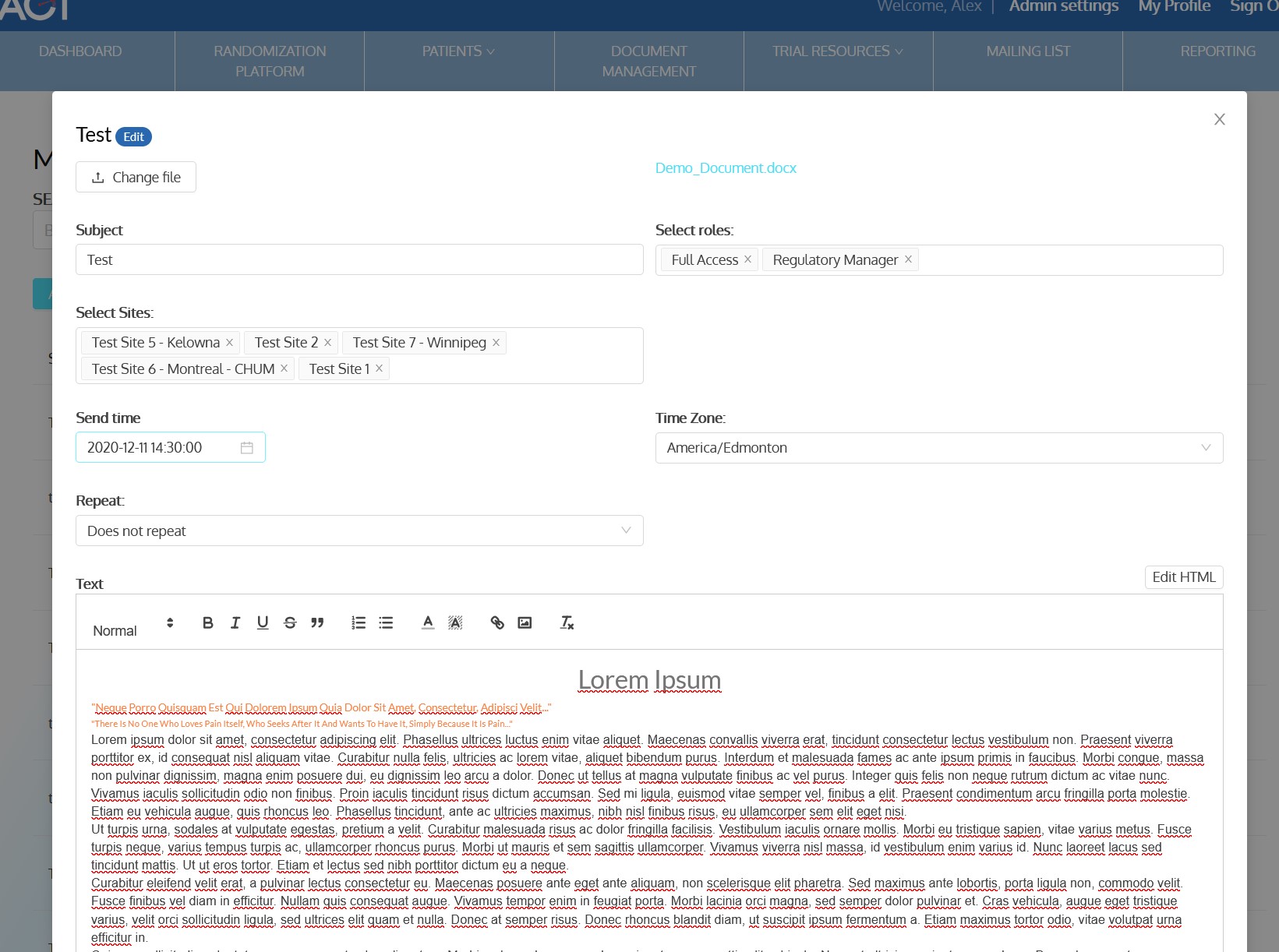
- Sends out periodic notifications to enrolled sites
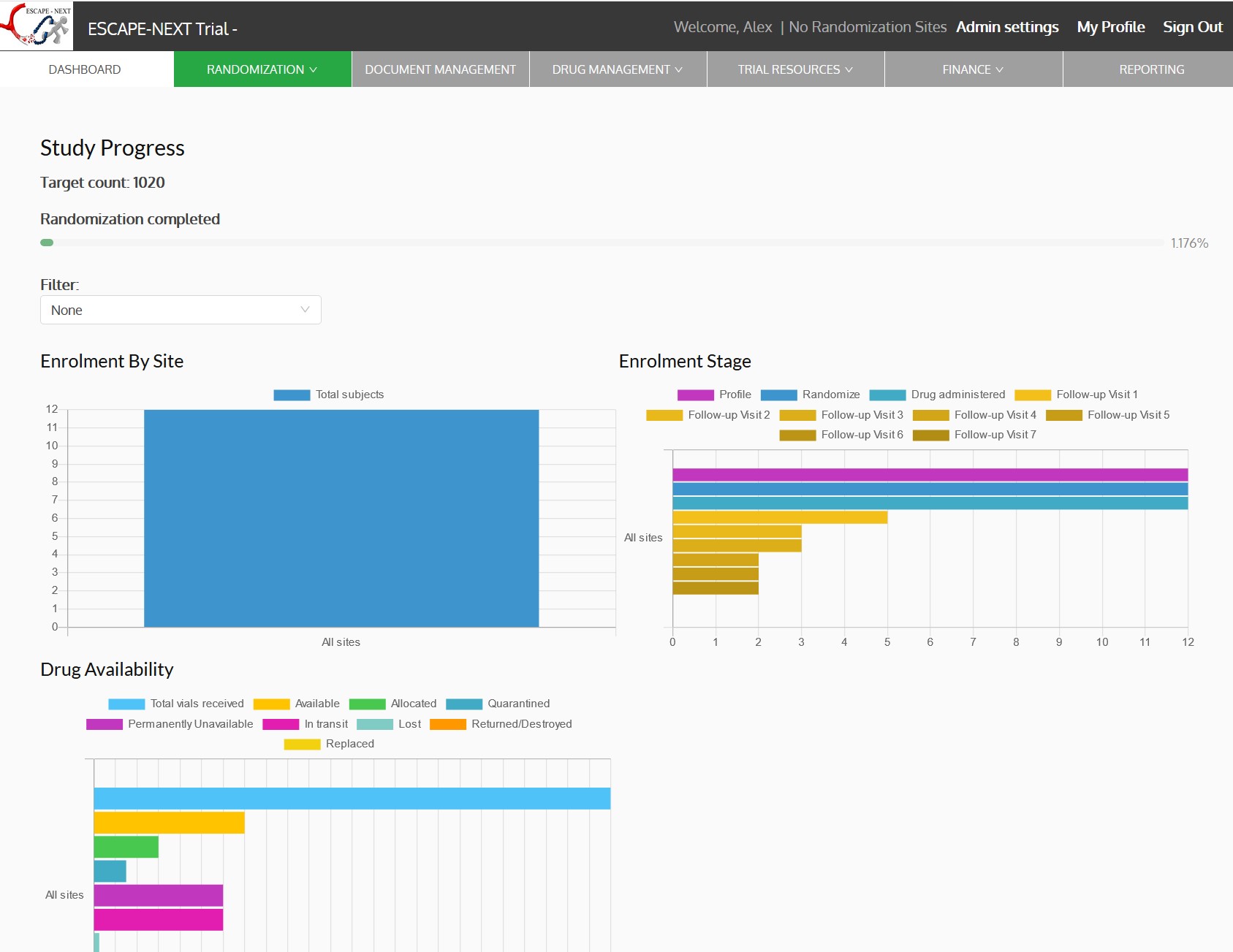
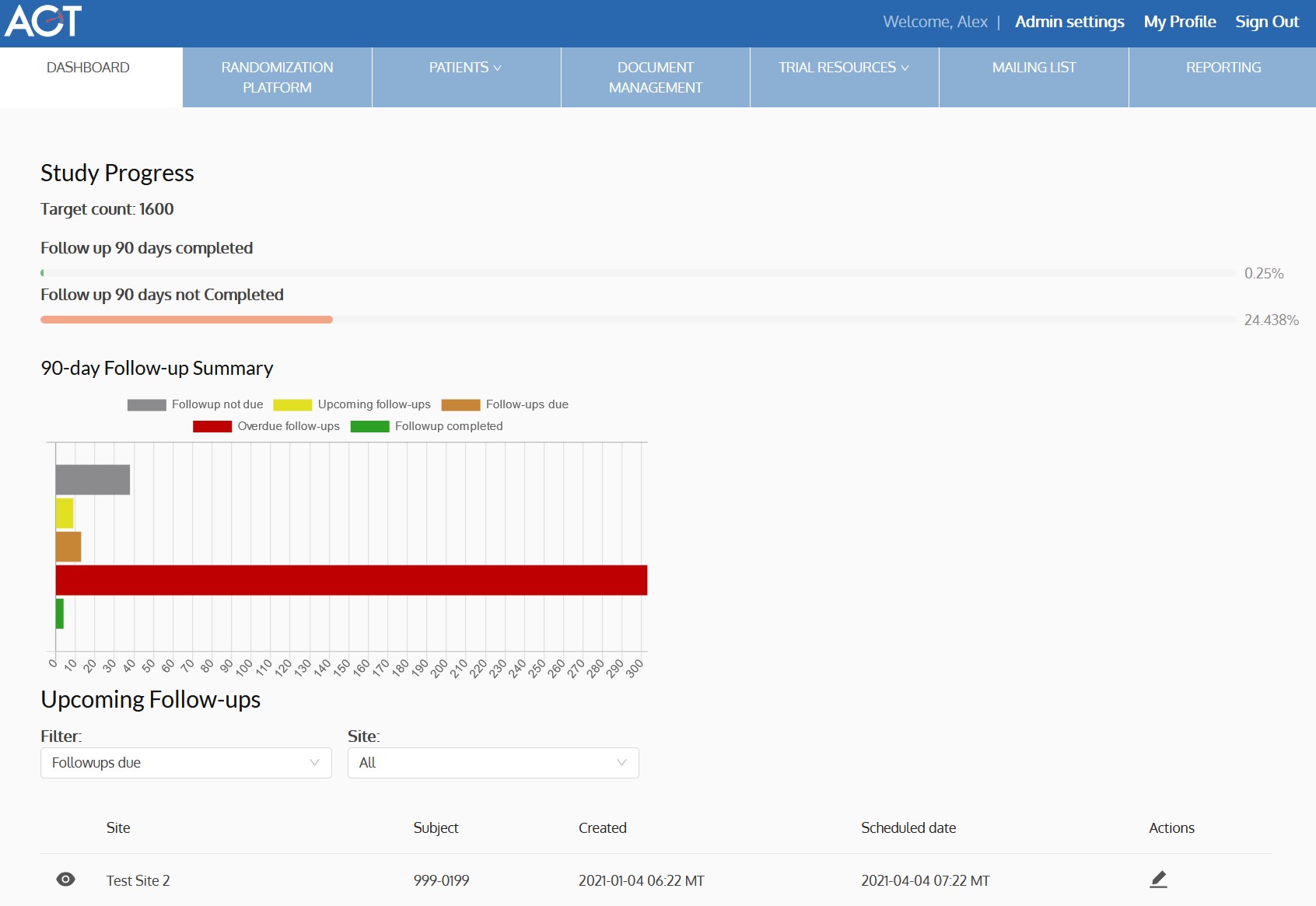
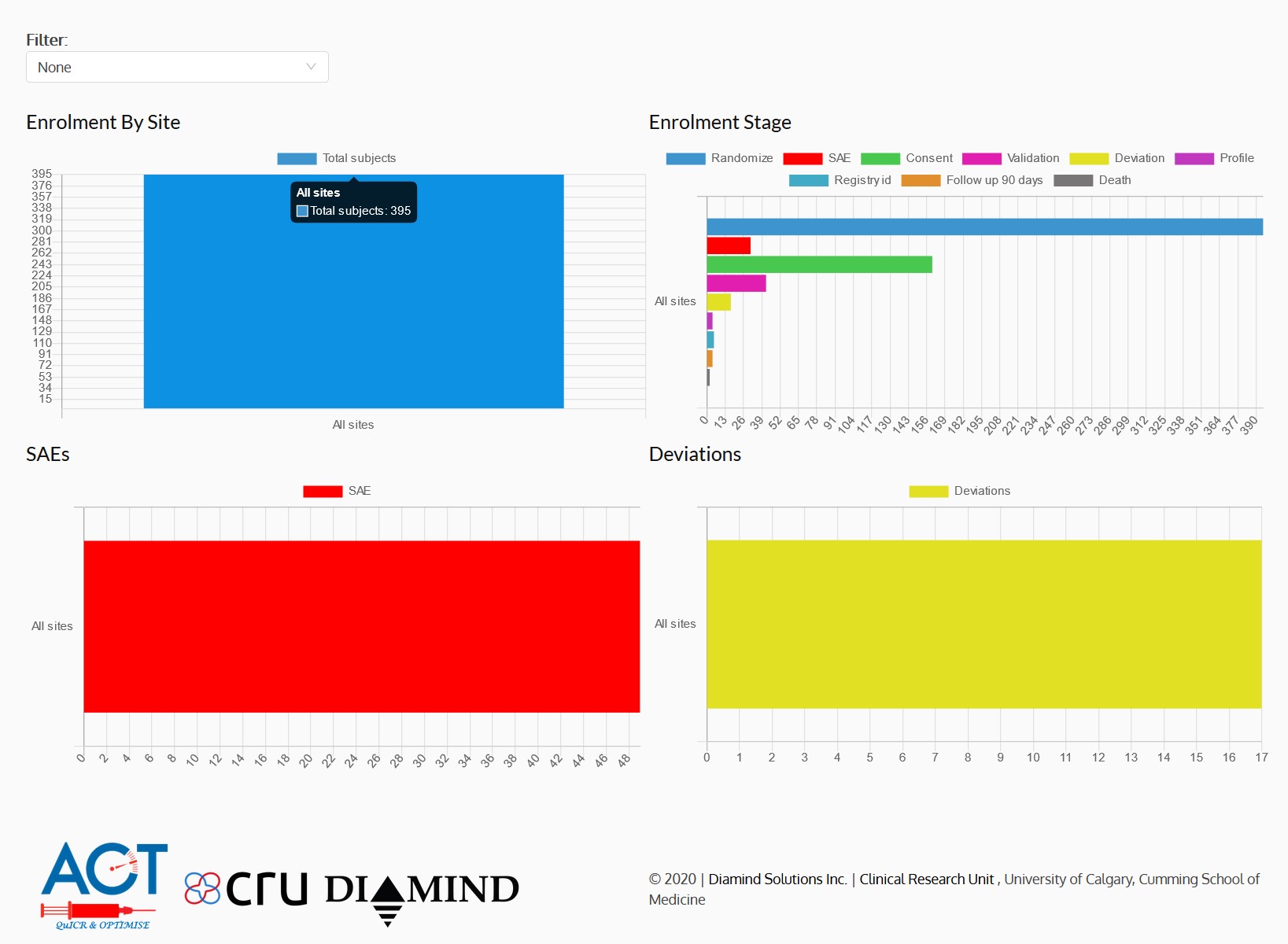
- Dashboards, charts and reports
- Secure & Scalable
Want to know more about DCTMS? Please visit our DCTMS Product page or contact us for a free demo!
Other Links:
Ready to enroll?Get started today!
Our main areas of expertise are in the Healthcare, Academic, Education as well as the Energy Sector, where we provide solutions for a number of needs:
- - Clinical Trial Management Solutions (CTMS)
- - Interactive Response Technologies (IRT)
- - Randomization & Drug Management
- - Document Management & eTMF
- - eConsent
- - EHR Integrations
- - Custom Application Development
- - Business Intelligence & Data Integration